(12分)实验室用下面装置测定FeO和Fe2O3固体混合物中Fe2O3的质量,D装置的硬质双通玻璃管中的固体物质是FeO和Fe2O3的混合物。
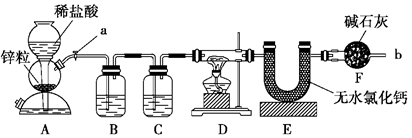
(1)如何检查装置A的气密性?
_
(2)装置A发生的反应有时要向其中加入少量CuSO4溶液,其目的是 ,
其原理是 。
(3)为了安全,在点燃D处的酒精灯之前,在b出口处必须 。
(4)装置B的作用是 ;
装置C中装的液体是 ,所起的作用是 。
(5)在气密性完好,并且进行了必要的安全操作后,点燃D处的酒精灯,在硬质双通玻璃管中发生反应的化学方程式是 。
(6)若FeO和Fe2O3固体混合物的质量为23.2 g,反应完全后U型管的质量增加7.2 g,则混合物中Fe2O3的质量为 g。
(7)U形管E右边又连接干燥管F的目的是 ,若无干燥管F,测得Fe2O3的质量将 (填“偏大”、“偏小”或“无影响”)。
(8)若反应后得到的残留固体中还有少量FeO,测得的Fe2O3质量将 (填“偏大”、“偏小”或“无影响”)。
(12分,每空1分) (1)关闭a,从球形漏斗口加水,待水从漏斗管上升与容器中的水面形成一段水柱,停止加水,静置片刻,如水柱不下降,证明其气密性良好
(2)加快氢气产生的速率 ;Zn先与CuSO4反应生成Cu附着在Zn表面,Zn(负极)、Cu(正极)与稀盐酸(电解质溶液)组成很多微小原电池,发生原电池反应,加快了反应速率
(3)检验氢气的纯度
(4)除去H2中混有的HCl气体 浓硫酸 干燥氢气
(5)Fe2O3+3H2 2Fe+3H2O,FeO+H2
2Fe+3H2O,FeO+H2 Fe+H2O
Fe+H2O
(6)16 (7)防止空气中的水蒸气等气体进入E中 偏大 (8)偏小
(1)A装置是启普发生器,所以检验气密性的方法是关闭a,从球形漏斗口加水,
待水从漏斗管上升与容器中的水面形成一段水柱,停止加水,静置片刻,如水柱不下降,证
明其气密性良好。
(2)由于Zn先与CuSO4反应生成Cu附着在Zn表面,Zn(负极)、Cu(正极)与稀盐酸(电解质溶液)组成很多微小原电池,发生原电池反应,从而加快了反应速率。
(3)氢气是可燃性气体,点燃之前必需检验氢气的纯度。
(4)由于生成的氢气中含有氯化氢和水蒸气,而这两种杂质都能干扰实验的,实验B中盛有饱和食盐水,用来除去H2中混有的HCl气体;而C中盛有浓硫酸,用来干燥氢气。
(5)在加热的条件小,氢气能还原金属氧化物,反应的方程式是Fe2O3+3H2 2Fe+3H2O,FeO+H2
2Fe+3H2O,FeO+H2 Fe+H2O。
Fe+H2O。
(6)设FeO和Fe2O3固体的物质的量分别是x和y,则72x+160y=23.2g。U形管增加的质量就是反应中生成的水的质量,因此有x+3y=7.2g÷18g//mol,解得x=y=0.1mol,实验氧化铁的质量是16g。
(7)由于空气中也含有水蒸气,所以F装置的作用是防止空气中的水蒸气等气体进入E中。如果没有F装置,则U形管增加的质量就偏大,所以测定结果偏高。
(8)若反应后得到的残留固体中还有少量FeO,则U形管增加的质量就偏小,所以测定结果偏小。

 缺失,下颌余留后牙严重舌向倾斜,采用铸造支架修复时所选择的大连接体最佳的是()
缺失,下颌余留后牙严重舌向倾斜,采用铸造支架修复时所选择的大连接体最佳的是()