某矿样含有大量的CuS及少量其它不溶于酸的杂质。实验室中以该矿样为原料制备CuCl2·2H2O晶体,流程如下:
(1)在实验室中,欲用37%(密度为1.19 g·mL-1)的盐酸配制500 mL 6 mol·L-1的盐酸,需要的仪器除量筒、烧杯、玻璃棒外,还有 、 。
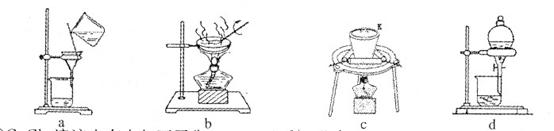
(2)①若在实验室中完成系列操作a。则下列实验操作中,不需要的是 (填下列各项中序号)。
②CuCl2溶液中存在如下平衡:Cu(H2O)42+(蓝色)+4Cl-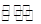 CuCl42-(黄色)+4H2O。
CuCl42-(黄色)+4H2O。
欲用实验证明滤液A(绿色)中存在上述平衡,除滤液A外,下列试剂中,还需要的是 (填下列各项中序号)。
a.FeCl3固体 b.CuCl2固体 c.蒸馏水
(3)某化学小组欲在实验室中研究CuS焙烧的反应过程,查阅资料得知在空气条件下焙烧CuS时,固体质量变化曲线及SO2生成曲线如下图所示。
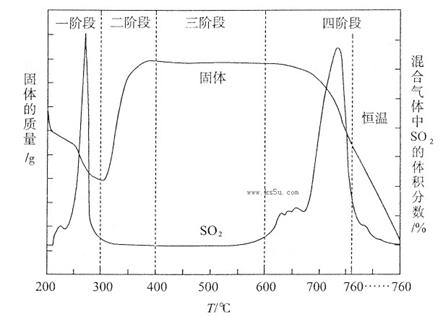
①CuS矿样在焙烧过程中,有Cu2S、CuO·CuSO4、CuSO4、CuO生成,转化顺序为:

第①步转化主要在200~300oC范围内进行,该步转化的化学方程式为 。
②300~400oC范围内,固体质量明显增加的原因是 ,上图所示过程中,CuSO4固体能稳定存在的阶段是 (填下列各项中序号)。
a.一阶段 b、二阶段 c、三阶段 d、四阶段
③该化学小组设计如下装置模拟CuS矿样在氧气中焙烧第四阶段的过程,并验证所得气体为SO2和O2的混合物。
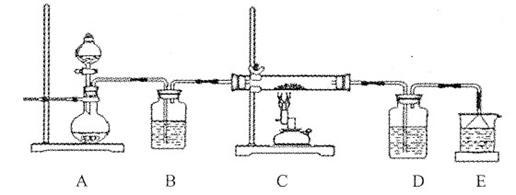
a.装置组装完成后,应立即进行的一项操作是 。
b.当D装置中产生白色沉淀时,便能说明第四阶段所得气体为SO2和O2的混合物。你认为装置D中原来盛有的溶液为 溶液。
c.若原CuS矿样的质量为l0.0 g,在实验过程中,保持温度在760oC左右持续加热,待矿样充分反应后,石英玻璃管内所得固体的质量为8.0 g,则原矿样中CuS的质量分数为 。
(1)(4分)胶头滴管(2分) 500mL容量瓶(2分)
(2)(4分)①cd(2分)②c(2分)
(3)(12分)①2CuS+O2=Cu2S+SO2(2分)②Cu2S转化为CuSO4(2分) c(2分)
③a.检验装置的气密性(2分) b.氯化钡(BaCl2)(2分) c.96%(2分)
题目分析:(1)由浓盐酸配制稀盐酸,需要的仪器除量筒、烧杯、玻璃棒外,还需要:胶头滴管、500mL容量瓶。
(2)①由CuCl2溶液制取CuCl2•2H2O,需要进行蒸发浓缩、降温结晶,然后过滤可得CuCl2•2H2O晶体,所以不需要c、d操作。
②FeCl3溶液为黄色,CuCl2溶液为蓝色,对原平衡体系溶液的颜色产生干扰,而加入蒸馏水可使平衡向逆反应方向移动,溶液的颜色发生改变,可证明滤液A(绿色)中存在上述平衡,故c项正确。
(3)①根据流程图CuS与O2反应生成Cu2S,还应生成SO2,化学方程式为:2CuS+O2=Cu2S+SO2
②第②步转化,由Cu2S转化为CuO•CuSO4,所以300~400oC范围内,固体质量明显增加的原因是Cu2S转化为CuSO4;第二阶段生成了CuSO4,第四阶段固体质量减小,CuSO4发生了分解反应,所以CuSO4固体能稳定存在的阶段是第三阶段。
③a.实验装置组装完后应首先检验装置的气密性;b. SO2与BaCl2不反应,SO2、O2与BaCl2溶液反应可以生成BaSO4沉淀,所以装置D中原来盛有的溶液为BaCl2溶液;c. 矿样充分反应后,石英玻璃管内所得固体CuO,根据Cu元素质量守恒,可知CuS ~ CuO ,所以CuS的质量为8g÷80g/mol×96g/mol=9.6g,则原矿样中CuS的质量分数为:9.6g÷10.0g×100%=96%
