问题
实验题
(1)向KAl(SO4)2溶液中滴加过量氨水,现象是____________________________,反应的离子方程式______________________________________________,再向其中加入过量NaOH溶液,反应现象是____________________________________________,反应的离子方程式为_______________________________________________。
(2)下列六个图中,横坐标为某溶液中加入某物质的物质的量,纵坐标为生成沉淀的物质的量,请把下表中各反应的相应图象的序号填入表中。
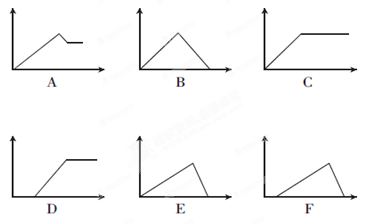
| 溶液 | 加入的物质 | 答案序号 |
| ①饱和石灰水 | 通过量CO2 | |
| ②AlCl3溶液 | 通过量NH3 | |
③MgCl2、AlCl3混合溶 液 液 | 逐滴加NaOH溶液至过量 | |
| ④AlCl3溶液 | 逐滴加NaOH溶液至过量 | |
| ⑤含少量HCl的AlCl3溶液 | 逐滴加NaOH溶液至过量 |
答案
(1) (1)生成白色胶状沉淀;Al3++3NH 3·H2O===Al(OH)3↓+3NH;白色胶状沉淀溶解,溶液变澄清;Al(OH)3+OH-===AlO+2H2O
3·H2O===Al(OH)3↓+3NH;白色胶状沉淀溶解,溶液变澄清;Al(OH)3+OH-===AlO+2H2O
(2)B;C;A;E;F(各1分)
