问题
填空题
现有下列四组溶液,每组包括三种溶液并按A、B、C顺序排列:
甲 乙 丙 丁
A.BaCl2 Na2CO3 KCl K2CO3
B.硝酸 硝酸 盐酸 盐酸
C.Na2CO3 AgNO3 K2CO3 BaCl2
请根据下列操作中所观察的现象完成下列问题:
向CaCl2溶液中加入A溶液产生白色沉淀;继续加入过量的B溶液沉淀消失,并产生无色无味的气体;再加入少量的C溶液,又生成白色沉淀。
(1)上述操作过程中,选用了 组溶液。
(2)有关反应的离子方程式是 。
答案
(1)乙
(2)Ca2++CO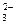
 CaCO3↓
CaCO3↓
CaCO3+2H+ Ca2++CO2↑+H2O
Ca2++CO2↑+H2O
Ag++Cl- AgCl↓
AgCl↓
向CaCl2溶液中加入A产生白色沉淀,四组溶液中乙、丁均产生CaCO3沉淀,继续加入过量B溶液,乙、丁均符合;再加入少量C溶液,又生成白色沉淀,则只有乙符合;因加入C之前,溶液中含有Cl-,与加入的Ag+反应产生AgCl沉淀。而丁不符合,因溶液中不含与Ba2+结合成沉淀的CO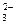 。
。
