水的电离平衡曲线如图所示:
(1)若以A点表示25℃时水的电离平衡时离子的浓度,当温度升高到100℃时,水的电离平衡状态到B点,则此时水的离子积从______增加到______.
(2)已知25℃时,0.1L0.1mo/L的NaA溶液的pH=10,则HA在水溶液中的电离方程式为______.
(3)100℃,将pH=9的NaOH溶液与pH=4的硫酸溶液混合,若所得混合溶液pH=7,则NaOH溶液与硫酸溶液的体积比为______.
(4)100℃时,若10体积的某强酸溶液与1体积的某强碱溶液混合后溶液呈中性,则混合之前,该强酸的pH与强碱的pH之间应满足pH酸+pH碱=______.
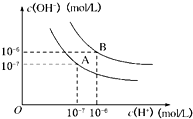
(1)水的离子积KW=c(H+)•c(OH-),25℃时,KW=c(H+)•c(OH-)=10-7×10-7=10-14;100℃时,KW=c(H+)•c(OH-)=10-6×10-6=10-12,
故答案为:10-14;10-12;
(2)由于0.1L 0.1mo/L的NaA溶液的pH=10,说明溶液显示碱性,HA属于弱电解质,电离方程式为:HA⇌H++A-,
故答案为:HA⇌H++A-;
(3)设氢氧化钠溶液的体积为xL,硫酸溶液的体积为yL,pH=9的NaOH溶液中,氢氧根离子的浓度为:10-3mol/L,pH=4的硫酸溶液中氢离子浓度为:10-4mol/L,
二者混合后溶液的pH=7,溶液酸性碱性,溶液中氢氧根离子的浓度为10-5mol/L,即氢氧化钠过量,即10-3mol/L×xL=10-4mol/L×y+10-5mol/L(x+y),
解得x:y=1:9,
故答案为:1:9;
(4)设强酸溶液的pH为a,体积为10V,溶液中氢离子浓度为:10-amol/L;碱溶液的pH为b,体积为V,溶液中氢氧根离子的浓度为:10-(12-b)mol/L,
混合后溶液呈中性,则满足溶液中氢离子的物质的量大于氢氧根离子的物质的量,即10-amol/L×10VL=10-(12-b)mol/L×VL,
解得:1-a=b-12,a+b=13,
故答案为:13.
