附加题:指错(下面每句话中有一处错误,从A、B、C、D中选出错误的一项,勿需改正。)
1. It was very kind for them to meet me at the railway station. ______
A B C D
2. Some people read the books and watch TV while others have sports. ______
A B C D
3. They will very happy when I tell them the news. ______
A B C D
4. I had to drive to home because my friend Bob had drunk too much beer. ______
A B C D
5. I'll have the car wait at the gate. Will that be all right? ______
A B C D
6. Tom and Jane went on do their homework till midnight. ______
A B C D
7. I want to thank you again for have me in your home for the holiday. ______
A B C D
8. That pair of trousers of yours are very dirty, you'd better clean it. ______
A B C D
9. Know some everyday English will be of great help. ______
A B C D
10. We hope you a pleasant trip back home. ______
A B C D
1-5 BAABB 6-10 BBCAA

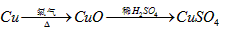

 以抑制水解)中取得产品的实验操作步骤应为_________ 、_______ 、__________ ,取得产品后的残留物质可循环使用。
以抑制水解)中取得产品的实验操作步骤应为_________ 、_______ 、__________ ,取得产品后的残留物质可循环使用。 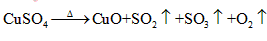 ,便决定设计实验测定反应生成的
,便决定设计实验测定反应生成的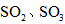 和O2的物质的量,并经计算确定该条件下
和O2的物质的量,并经计算确定该条件下 分解反应方程式中各物质的化学计量数。试验可能用到的仪器如下图所示:
分解反应方程式中各物质的化学计量数。试验可能用到的仪器如下图所示: