(8分)短周期元素A、B、C、D在周期表中位置如图所示,其中元素D原子最外层有3个电子。
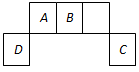
(1)A与氢元素可以形成很多化合物,在这些化合物中都含有 (填“共价”或“离子”)键;B在元素周期表中位于 族;
(2)B、C、D形成的简单离子的半径由大到小依次为 。(填相应的离子符号)
(3)D的最高价氧化物对应的水化物与NaOH溶液反应的化学方程式是 。
(4)已知298K时,Fe(s) + 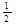 O2(g) ="=" FeO(s) ΔH= -300.0 kJ·mol-1
O2(g) ="=" FeO(s) ΔH= -300.0 kJ·mol-1
2D(s) + 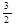 O2(g) ="=" D2O3(s) ΔH= -1675.7 kJ·mol-1
O2(g) ="=" D2O3(s) ΔH= -1675.7 kJ·mol-1
写出D单质和FeO反应的热化学方程式是 。
(8分)(1)共价键; VA族;(各1分) (2)Cl->N3->Al3+(2分)
(3)Al(OH)3+NaOH=NaAlO2 +2H2O(2分)
(4)2Al(s)+3FeO(s)=Al2O3(s)+3Fe(s) ΔH= -775.7kJ·mol-1(2分)
题目分析:D元素的最外层电子数是3个,根据D的位置可判断,D是第三周期元素。所以D是Al,则A是C、B是N、C是Cl。
(1)碳元素形成的氢化物中都含有C-H极性键;氮元素属于第ⅤA族。
(2)核外电子排布相同的微粒,其微粒半径岁原子序数的增大而减小,所以B、C、D形成的简单离子的半径由大到小依次为Cl->N3->Al3+。
(3)氢氧化铝是两性氢氧化物,能和氢氧化钠溶液反应,化学方程式是Al(OH)3+NaOH=NaAlO2 +2H2O。
(4)根据盖斯定律可知,②-①×3即得到2Al(s)+3FeO(s)=Al2O3(s)+3Fe(s),所以该反应的反应热是△H=-1675.7kJ/mol+300.0kJ/mol×3=-775.7kJ/mol。
点评:本题主要是元素“位、构、性”三者关系的综合考查,比较全面考查学生有关元素推断知识和灵活运用知识的能力。该题以“周期表中元素的推断”为载体,考查学生对元素周期表的熟悉程度及其对表中各元素性质和相应原子结构的周期性递变规律的认识和掌握程度。考查了学生对物质结构与性质关系以及运用元素周期律解决具体化学问题的能力。
