During our two months on the road, Bennett and I had a really (1) experience with a good, honest (2) and some helpful mechanics.
We were driving east on Highway 10 when our "chick engine" light came on. We limped of a(n) (3) into Las Cruces. We had a real car (4) Bennett nursed the ear into a local garage. By this time the car was missing so (5) it was shaking all over. This was the (6) time to arrive at a garage—late Friday afternoon. Service adviser Scott was busy (7) paper work and customers as we (8) our problems. (9) he was already "ten cars behind", he told us to pull the car into the garage.
Lincoln, who we later (10) was one of the two motor technicians, took (11) of our car repairing. He and Scott and some other mechanics stayed several hours after closing, (12) the car.
Early the next morning (the shop was officially closed on Saturdays), Lincoln finally located the (13) and fixed it easily within only (14)
Later Scott (15) out to us that it was our attitude that helped. "You didn’t come into the place demanding this or that. You showed an (16) of our problems on a busy Friday afternoon. Customer’s attitude means a lot. " He was right in some way, customers should show (17) and understanding to people who (18) them. (19) people were extremely busy, they found way to at least try and help when they are met with politeness.
The pleasant experience I had shows that (20) for other people can always help.
6().
A.highest
B.easiest
C.luckiest
D.worst
参考答案:D

 CaCO3
CaCO3 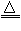 2MgO+C
2MgO+C 
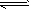 CH3OH(g)。某研究小组将2 mol
CH3OH(g)。某研究小组将2 mol