氮可以形成多种离子,如N3-,NH2-,N3-,NH4+,N2H5+,N2H62+等,已知N2H5+
与N2H62+是由中性分子结合质子形成的,类似于NH4+,因此有类似于 NH4+的性质。
⑴写出N2H62+在碱性溶液中反应的离子方程式 。
⑵NH2-的电子式为 。
⑶N3-有 个电子。
⑷写出二种由多个原子组成的含有与N3-电子数相同的物质的化学式 、 。
⑸等电子数的微粒往往具有相似的结构,试预测N3—的构型 。
⑹据报道,美国科学家卡尔·克里斯特于1998年11月合成了一种名为“N5”的物质,由于其具有极强的爆炸性,又称为“盐粒炸弹”。迄今为止,人们对它的结构尚不清楚,只知道“N5”实际上是带正电荷的分子碎片,其结构是对称的,5个N排成V形。如果5个N结合后都达到8电子结构,且含有2个N≡N键。则“N5”分子碎片所带电荷是 。
⑴N2H62++2OH-=N2H4+2H2O ⑵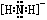 ⑶22
⑶22
⑷N2O CO2 CNO- BeF2 CaH2 C3H4等 ⑸直线型 ⑹一个单位正电荷
题目分析:⑴NH4+与碱反应的离子方程式为:NH4++OH-=NH3+H2O。由于NH4+与N2H62+结构相似,所以N2H62+在碱性溶液中反应的离子方程式为N2H62++2OH-=N2H4+2H2O。⑵NH2-是NH3失去质子后形成的。所以NH2-的电子式为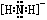 。⑶N是7号元素,所以N3-有22个电子。⑷由多个原子组成的含有与N3-电子数相同的物质有N2O 、 CO2、 CNO-、BeF2、CaH2、C3H4等。⑸等电子数的微粒往往具有相似的结构,由于CO2与N3—是等电子体。所以N3—的构型是直线型。⑹其结构是对称的,5个N排成V形。如果5个N结合后都达到8电子结构,且含有2个N≡N键。其结构为
。⑶N是7号元素,所以N3-有22个电子。⑷由多个原子组成的含有与N3-电子数相同的物质有N2O 、 CO2、 CNO-、BeF2、CaH2、C3H4等。⑸等电子数的微粒往往具有相似的结构,由于CO2与N3—是等电子体。所以N3—的构型是直线型。⑹其结构是对称的,5个N排成V形。如果5个N结合后都达到8电子结构,且含有2个N≡N键。其结构为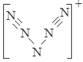 “N5”分子碎片所带电荷是一个单位正电荷。
“N5”分子碎片所带电荷是一个单位正电荷。
