问题
填空题
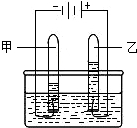
有关电解水实验(如右图),试回答:,
(1)试管甲中的气体是______;
(2)试管乙中的气体是______检验该气体的方法______;
(3)试管甲和试管乙所收集到的气体体积比为:______;
(4)通过该实验,我们可以得出水由______组成的结论.
答案
(1)甲试管中生成的气体较多是氢气.
(2)乙试管中生成的气体较少是氧气,能使带火星的木条复燃.
(3)氢气与氧气的体积比是2:1.
(4)根据以上现象可推出正极产生的气体是氧气,负极产生的气体是氢气,还能进一步推出水由氢元素和氧元素组成.
故答案为:氢气;氧气;将带火星的木条深入使观众该木条复燃,该气体就是氧气;2:1;氢、氧两种元素.
