(6分)某有机化合物A的相对分子质量(分子量)大于110,小于150。经分析得知,其中碳和氢的质量分数之和为52.24%,其余为氧。请回答:
(1)该化合物分子中含有几个氧原子,为什么?
(2)该化合物的相对分子质量(分子量)是 。
(3)该化合物的化学式(分子式)是 。
(4)该化合物分子中最多含 个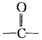 官能团。
官能团。
(1)4;(2)134;(3)C5H10O4;(4)1
⑴由题意知,O的质量分数为47.76%,由化合物A的相对分子质量大于110,小于150,
所以氧原子个数为大于110×0.4776/16=3.28,小于150×0.4776/16=4.48,所以氧原子为4个,
⑵所以有机物分子质量=4×16/0.4776=134,其中C、H的相对原子质量之和为134-16×4=70,可确定分子式为C5H10O4. C5H10O4与5个C原子的饱和衍生物(可表示为C5H12On)比较可知,分子中最多含有1个羰基官能团.
故答案为:(1)4;(2)134;(3)C5H10O4;(4)1;.
