地之都,水绿盐城!
(1)新四军纪念馆是盐城市的名片之一。馆内有许多抗战时的枪炮,但有些表面出现锈迹,
其主要原因是:铁与________、________等物质共同作用的结果。
(2)郭猛的温泉度假村是人们休闲的好去处。
①温泉水富含钾、钙、镁、氟、硅等,这里的“钾、钙、镁、氟、硅”指的是。____(填标号)
A.分子 B.原子 C.元素 D.单质
②温泉水的pH在7.5~8.9之间,该温泉水显______(填“酸性”、“碱性”或“中性”)。
③检验该温泉水是硬水还是软水的简单方法是____________________________________。
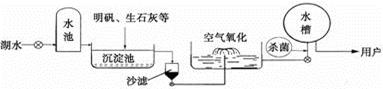
(3)新建的龙冈盐龙湖是一项重大的惠民工程,将为盐城人民提供优质水源。现在自来水的
生产过程如上图所示。
①下列属于自来水生产使用的净水方法是______。(填标号)
A.沉淀 B.过滤 C.煮沸 D.蒸馏
②自来水可用氯气杀菌消毒。请将下列化学方程式补充完整: Cl2 + H2O =" HClO" +________。
③能否用硝酸银区分盐城的自来水和蒸馏水_________。(填“能”或“不能”)
(4)盐城是一座以“盐”命名的城市,有着独特、深远的海盐文化,有着丰富的海水资源。以
下是我市对海水资源的部分利用。
①从海水中获得氯化钠。将海水进行 可得到粗盐
②粗盐可以用过溶解、________、蒸发等操作除去其中的难溶性杂质如泥沙。
③粗盐中含有的:CaSO4、MgCl2等可溶性杂质,有如下操作:①溶解;②加过量的Na2CO3 溶液; ③加过量的BaCl2溶液;④加适量的盐酸;⑤加过量NaOH溶液;⑥蒸发结晶;⑦过滤。正确的操作顺序是 。(用序号填空)
A、①⑤②③④⑦⑥ B、①③②⑤④⑦⑥ C、①③⑤②⑦④⑥
④氯化钠除食用外还是一种化工产品,如工业上利用氯化钠和水在通电条件下生成烧碱、氢气和氯气。请写出该反应的化学方程式 ________________________________________。
⑤从海水中得到金属镁。下图是从海水中提取镁的简单流程。
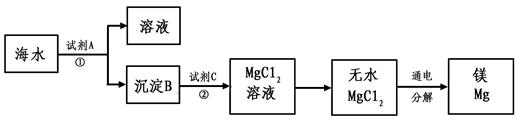
上述过程中,试剂A为熟石灰,则沉淀B为____________________。请写出沉淀B与试剂C 发生的反应的化学方程式 。
⑴氧气;水 ⑵①C ②碱性 ③用肥皂水搅拌 ⑶①A、B ②HCl ③能
⑷①蒸发 ②过滤 ③C ④2NaCl+2H2O 通电 2NaOH+H2↑+Cl2↑
⑤Mg(OH)2;Mg(OH)2+2HCl=MgCl2+H2O
题目分析:(1)钢铁生锈的原因是钢铁与氧气和水共同作用的结果,缺一不可,酸性溶液、碱性溶液、盐溶液能促进金属生锈。
(2)①物质是由元素组成的,温泉水中含有的“钾、钙、镁、氟、硅”等,是以化合物的形式存在于温泉水中的,不能以分子、原子、单质的形式存在,故是指元素。故选C
②当溶液的pH等于7时,呈中性;当溶液的pH小于7时,呈酸性;当溶液的pH大于7时,呈碱性;根据题意,温泉水的pH在7.5~8.9之间,大于7,故该温泉水显碱性。
③检验硬水和软水,要用肥皂水,可在等量的水样中分别加入等量的肥皂水振荡,泡沫多的是软水,泡沫少的是硬水。
(3)①自来水生产的过程中,常用的净水方法有沉淀、过滤、吸附等。故选AB
②由反应的化学方程式Cl2 + H2O =" HClO" +________可知,
反应前 反应后
Cl原子 2 1
H原子 2 1
O原子 1 1
根据质量守恒定律,化学变化前后原子的种类、数目不变,可判断另一生成物分子中含有1个H原子和1个Cl原子,则该物质的化学式为HCl。
③根据第②题可知,自来水中含有Cl-,故向自来水中加硝酸银会生成白色沉淀;而蒸馏水是纯净物,故向其中加硝酸银无明显现象,二者现象不同,可以鉴别。
(4)①因为氯化钠的溶解度随温度变化不大,所以海水制盐常用蒸发结晶法,即蒸发海水达到饱和,再继续蒸发即可得到粗盐。
②根据粗盐提纯的过程分析,粗盐提纯的过程是溶解、过滤、蒸发、计算。
③要除去粗盐中含有的可溶性杂质,在加入除杂试剂的时候,碳酸钠必须加在氯化钡的后面,以保证将过量的钡离子除掉。先将粗盐加水溶解①,然后加过量的BaCl2溶液③,反应生成硫酸钡沉淀而去除硫酸根离子;加过量NaOH溶液⑤,反应生成氢氧化镁沉淀而去除镁离子;再加过量的Na2CO3 溶液②,反应生成碳酸钙沉淀和碳酸钡沉淀而去除钙离子及BaCl2中的钡离子;过滤⑦掉沉淀之后,再加适量的盐酸④,反应除去过量的NaOH,Na2CO3;最后蒸发结晶⑥即可,其中③⑤顺序可以颠倒。故选C
④根据题意,氯化钠和水在通电条件下生成烧碱、氢气和氯气,故该反应的化学方程式为2NaCl+2H2O 通电 2NaOH+H2↑+Cl2↑。
⑤在含有氯化镁的海水中加入氢氧化钙,二者能反应生成氢氧化镁沉淀和氯化钙,故沉淀B为Mg(OH)2;氢氧化镁沉淀只能与盐酸反应,才能生成氯化镁,故反应的化学方程式为Mg(OH)2+2HCl=MgCl2+H2O。-的检验,氯化钠与粗盐提纯,物质除杂或净化的探究,实验步骤的探究,书写化学方程式
点评:本题综合考查了海水中化学资源的利用,涉及的知识点繁多,除了要求学生在平时的学习中,要加强“双基”的储备与巩固之外,还要做到仔细审题,认真解答,只有这样才能正确作答。
